Biosergen has received approval for the next step in its phase 1 study of its drug against deadly fungal infections, which has blockbuster potential. Not a big price trigger, but one is approaching.
Biosergen: 1.5 million people die each year from fungal infections. Biosergen, which has FDA orphan drug status, is developing an antifungal drug that is more effective and has a significantly better side-effect profile than existing antifungal drugs developed 40-70 years ago. With USD 16.7 billion spent annually on antifungal drugs, Bioserg’s drug has blockbuster potential (annual sales of more than USD 1 billion) and is expected to be launched in Q2 2026.
Biosergen: Ticker: BIOSGN | Price: SEK 1,21 | Market Cap.: 51 MSEK | YTD price development: 15%
A study that went according to plan
Biosergen’s phase 1 study, to confirm that the drug is safe and without the same serious side effects as existing drugs, has been running according to plan. The first part of the study (Single Ascending Dose), in which the dose was gradually increased for each new group of subjects, has been completed and the results will be published soon. The next part of the study (Multiple Ascending Dose), subjects receive the drug every 7 days at progressively higher doses. The aim is to find the appropriate dose level. This is where Biosergen has been given permission to start the second of five groups of subjects, a significant positive step in the development of the drug.
Price Triggers
Approval to continue the phase 1 MAD study is positive, but probably not news that will have a major effect on the share price in the short term. However, the results of the phase 1 SAD study could be, while continued positive news from the MAD part of the study could have a long-lasting positive effect on the share price.
Triggers going forward
- Top-line results from Phase I study with SGN005 (SAD) Q1 2023
- Continued progression in SGN005 study 2023
- Top-line results from Phase I study with SGN005 (MAD) 2023
- Presentation of data to FDA and discussion of Phase II study Q2 2023
Read more: Investmentcase for Biosergen
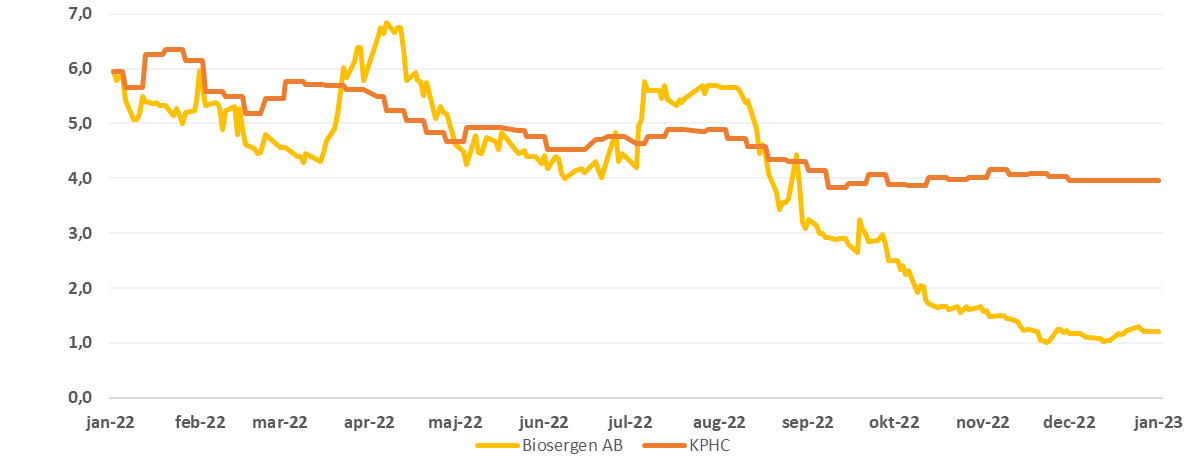
Price development of Biosergen 1 year vs. Kapital Partner Healthcare Index (KPHC)