Curasight has received positive results from a Phase 2 study of uTRACE in aggressive brain cancer, validating the technology and opening up combination therapies with uTREAT. In addition, it paves the way for new partner agreements – including a combined partner agreement for both uTRACE and uTREAT (theranostic platform) for glioblastoma.
Curasight: Ticker: CURAS | Price: DKK 21.8 | Market Cap: 446 MDKK | YTD price development: 114%
Curasight, a phase 2 biotech company within PET (Positron Emissions Tomography) and Radionuclide Therapy, has developed two products that can identify cancer cells (uTRACE) and target radiation therapy (uTREAT) to cancer cells. The advantage of Curasight’s products is that they avoid irradiating healthy tissue, which is a major side effect of traditional radiation therapy. Curasight avoids this by using uTREAT to bind to receptors that are only found in cancer cells and show where the cancer cells are. By adding a radiation source (uTREAT), radiation is delivered directly to the cancer cells, preventing healthy tissue from receiving radiation. Overall, this is a theranostic platform.
Positive phase 2 results indicate potential for combination therapy
Curasight has received positive study results in its Phase 2 study of uTRACE in aggressive brain cancer (glioblastoma). The study demonstrated that uPAR-PET/MR is suitable for the detection and prognosis of glioblastoma. 24 patients were included in the study, of which 94% had uPAR-PET positive tumors. This indicates that 94% of them may be suitable for future uPAR-targeted treatment with e.g. uTREAT. This is great news for Curasight as it suggests that they can use uTRACE and uTREAT in combination with each other. In other words, Curasight can use uTRACE to identify patients suitable for treatment and then use uTREAT to deliver radiation to the patients’ cancer cells.
Another validation of the technology paves the way for new partner agreements
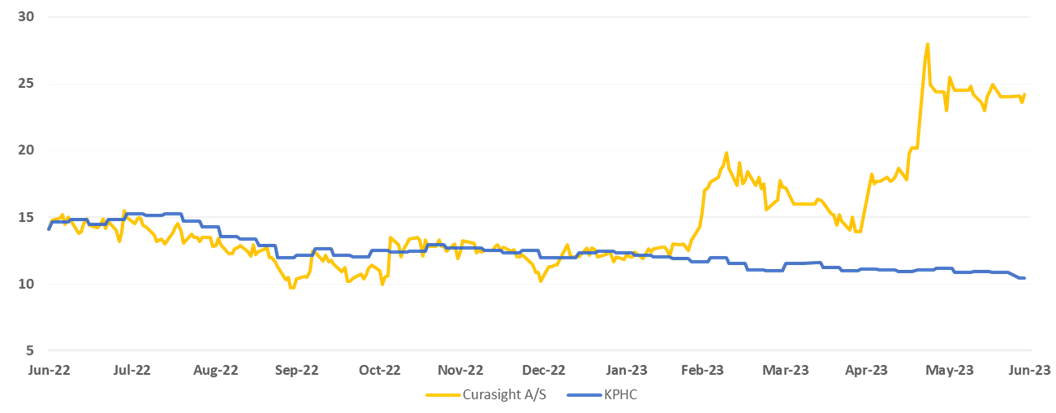
The Phase 2 study further validates Curasight’s technology platform following positive preclinical results with uTREAT earlier this month, increasing the likelihood of future market approval. In addition, Curasight entered into an agreement with Curium earlier this year for uTRACE in prostate cancer, where Curasight will receive up to USD 70 million in milestone payments and royalties on sales. Today’s positive study results open the door for Curasight to enter into a similar agreement with uTRACE for glioblastoma. Perhaps the company could even do a single partnering deal for both uTRACE and uTREAT for glioblastoma since the company received positive preclinical results with uTREAT for glioblastoma in early June. New partner agreements act as price trigger events that could provide a further boost to the share price, which has risen 88% in the past year and 131% year-to-date. Curasight has significantly outperformed the KPHC index, which tracks all Nordic healthcare companies.
Price development of Curasight vs. Kapital Partner Healthcare Index over the past year