After CS Medica recently obtained positive results from a clinical study with its CBD pain patch, CS Medica has now also received approval in Israel for sale in the retail market (OTC). It is expected that CS Medica’s other CBD-based products will also be approved in Israel, so that the entire product range can be sold in Israel.
CS Medica: Ticker: CSMED | Price: DKK 6.44 | Market Cap: DKK 84 million | YTD price development: -42%.
CS Medica is a Danish MedTech/biotech company focusing on, among other things, the treatment of pain, stress and autoimmune diseases with CBD (non-euphoriant substance from the cannabis plant) as a primary ingredient. The products are sold under the brand name CANNASEN to e.g. pharmacies and retail chains such as Matas, and as OEM to pharmaceutical companies and manufacturers. Several of CS Medica’s products are probably the only CBD products registered as “medical devise” under the European MDD directive, which gives the company a first mover advantage in CBD-based products.
Long-term share price effect
Pain Patch is one of CS Medica’s CBD-based products, all of which are sold at retail (OTC). The approval in Israel opens up a market that is among the leading countries in terms of sales of cannabis-based products, including CBD. CS Medica has not disclosed when the rest of its product range will be approved in Israel, which could make a significant contribution to the company’s revenue.
The price impact of the news is probably modest in itself, but the approval strengthens the investment case for sales in more and more countries and thus the long-term share price.
In the short term, the share price is still primarily linked to news about CS Medica’s Chinese activities, including the joint venture and potential investment from China’s RongShi, which over the past 12 months has resulted in very large price fluctuations.
Read more: The investment case and price triggers for CS Medica
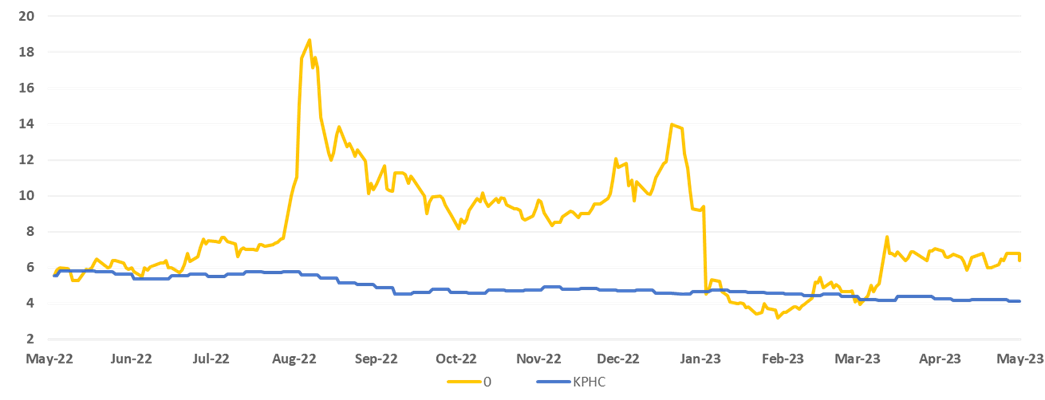
Price development for CS Medica A/S vs. Kapital Partner Healthcare Index the past year