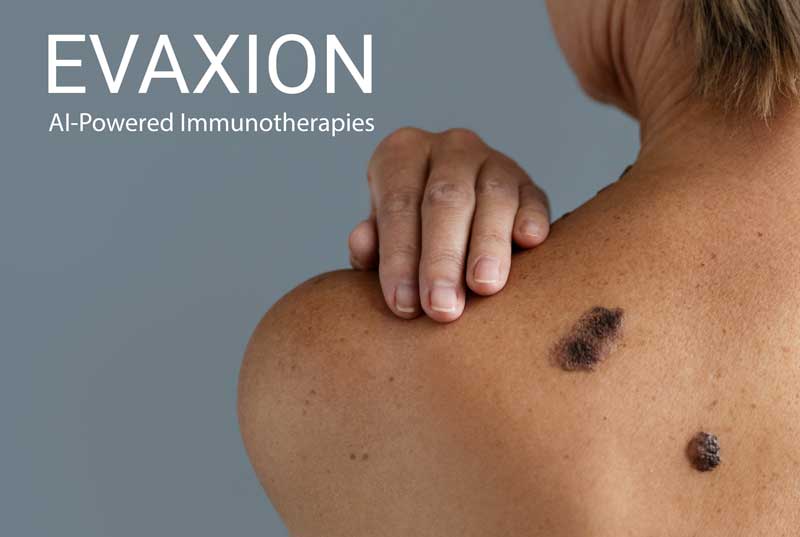
In Denmark alone, 270 people die each year from birthmark cancer, the most common cancer in the 15-34 age group, and 2,500 new cases are registered each year. It costs Danish society alone 250 million DKK. – every year. For this reason alone, Evaxion Biotech is an interesting company that is currently enrolling the first patients in its Phase IIb study.
US-listed Danish biotech company Evaxion Biotech [EVAX – price 3.39] has published its half-year report for 2022. It contains – for regulatory reasons – nothing new, but is a good reason to summarise the investment case.
USD 25 million in cash
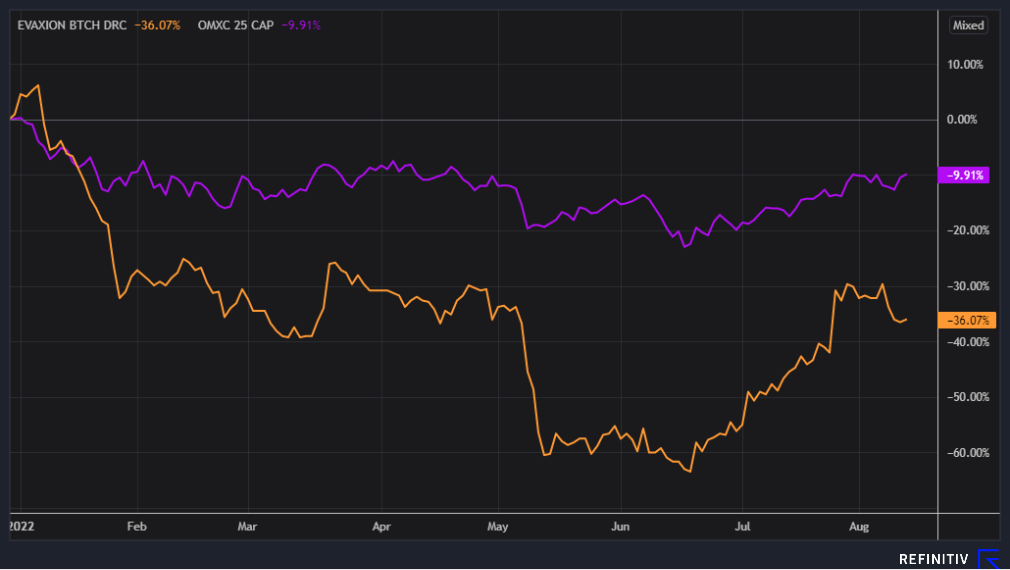
Evaxion Biotech has USD 25 million in cash on hand, which extends beyond 12 months, and has USD 40 million in back-up financing. It is therefore not a company that needs to raise capital, which puts Evaxion Biotech in a significantly better position than many other biotech companies. Nevertheless, the share price has fallen with the market, but has shown very good progress over the summer.
Price development year-to-date: Evaxion Biotech & C25 index
Partner in sight
The business model is to out-license the projects to a partner/pharmaceutical company once the projects are through Phase II. The initiation of the Phase IIb study with the EVX-01 programme (treatment of metastatic/spreading melanoma) in combination with Merck’s KEYTRUDA inhibitor could be the first programme to be out-licensed – and Merck would be an obvious partner.
There’s an interesting news flow ahead
In addition to the Phase IIb study, news on regulatory filings for new programmes is expected in this half year and data from the Phase 1/2a study for the EVX-02 programme in patients with mantle cell cancer who have undergone surgery is expected in H1 2023.
Expansion of the pipeline
Molecular cancer is currently the mainstay of the investment case in Evaxion Biotech, but the pipeline is being expanded with new indications. These include the EVX-03 programme for the treatment of lung cancer and, most recently, the EVX-B2 programme for the treatment of gonorrhoea.